Course
Biochemistry
Study Pack
Set 2 The Matrix Of Life Weak Interactions In An Aqueous Environment
Question 1
(Multiple Choice)
Free
If gastric juice has a pH of about 1.5,which of the following would be predominantly deprotonated in the stomach?
A)phenol (pKa = 9.9)
B)acetic acid (pKa = 4.7)
C)lactic acid, (pKa = 3.9)
D)phosphoric acid (pKa = 2.1)
E)hydrochloric acid (pKa = -6)
Answer
Question 2
(Multiple Choice)
Free
Which of the following would likely form micelles in an aqueous solution?
A)hexane
B)glucose
C)glutamic acid
D)dodecanoic acid
E)none of the above
Answer
Question 3
(Multiple Choice)
Free
In the following titration curve,what does the inflection point represent? 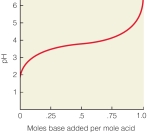

A)pH of solution equals pKa of weak acid
B)concentration of weak acid and conjugate base are equal
C)the pH where the solution would function most effectively as a buffer
D)the weak acid is 50% protonated,50% deprotonated
E)all of the above
Answer