Question 3
(Multiple Choice)
In the following titration curve,what does the inflection point represent? 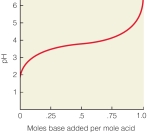
A)pH of solution equals pKa of weak acid
B)concentration of weak acid and conjugate base are equal
C)the pH where the solution would function most effectively as a buffer
D)the weak acid is 50% protonated,50% deprotonated
E)all of the above
Answer
E