Course
Microbiology
Study Pack
Set 13 Energetics And Catabolism
Question 1
(Multiple Choice)
Free
Glycolytic reactions with a near-zero Δ G°' can participate in the overall pathway of gluconeogenesis because they
A) are irreversible.
B) are reversible.
C) contradict the laws of thermodynamics.
D) have low energy of activation values.
E) do not depend on concentrations of reactants and products.
Answer
Question 2
(Multiple Choice)
Free
A metabolic process allowing for anaerobic degradation of benzoate to acetate is diagrammed below. This metabolism can happen because 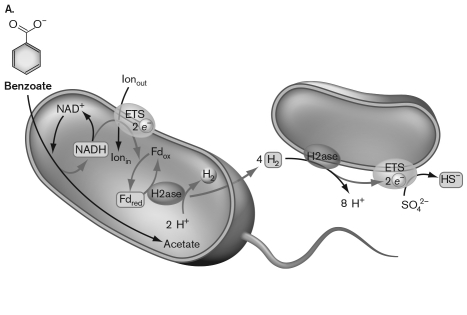 Δ G of H2production. E) the cell on the left swims away from the H2that it produces in order to balance the thermodynamics." class="answers-bank-image d-block" loading="lazy" >
Δ G of H2production. E) the cell on the left swims away from the H2that it produces in order to balance the thermodynamics." class="answers-bank-image d-block" loading="lazy" >
 Δ G of H2production. E) the cell on the left swims away from the H2that it produces in order to balance the thermodynamics." class="answers-bank-image d-block" loading="lazy" >
Δ G of H2production. E) the cell on the left swims away from the H2that it produces in order to balance the thermodynamics." class="answers-bank-image d-block" loading="lazy" >A) benzoate contains a great deal of energy and ATP can be made.
B) the catabolism of benzoate by the cell on the left produces hydrogen that is then used by the cell on the right to reduce sulfate.
C) the catabolism of benzoate by the cell on the left produces hydrogen that is then used by a methanogen.
D) the catabolism of benzoate to acetate releases so much energy that it overcomes the positive Δ G of H2 production.
E) the cell on the left swims away from the H2 that it produces in order to balance the thermodynamics.
Answer
Question 3
(Multiple Choice)
Free
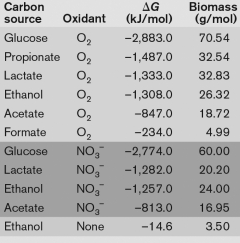
Using the table below, what is the best method for obtaining energy from catabolizing ethanol, and why? A.
 Δ G is almost positive B) the oxidation of ethanol with NO3− as the oxidant is because of the large yield of energy released C) the oxidation of ethanol with oxygen, because the Δ G is the largest negative number indicating the large yield of energy released. D) The oxidation of ethanol yields too little biomass to be catabolized. E) None-ethanol can only be catabolized syntrophically." class="answers-bank-image d-inline" loading="lazy" >
Δ G is almost positive B) the oxidation of ethanol with NO3− as the oxidant is because of the large yield of energy released C) the oxidation of ethanol with oxygen, because the Δ G is the largest negative number indicating the large yield of energy released. D) The oxidation of ethanol yields too little biomass to be catabolized. E) None-ethanol can only be catabolized syntrophically." class="answers-bank-image d-inline" loading="lazy" >
 Δ G is almost positive B) the oxidation of ethanol with NO3− as the oxidant is because of the large yield of energy released C) the oxidation of ethanol with oxygen, because the Δ G is the largest negative number indicating the large yield of energy released. D) The oxidation of ethanol yields too little biomass to be catabolized. E) None-ethanol can only be catabolized syntrophically." class="answers-bank-image d-inline" loading="lazy" >
Δ G is almost positive B) the oxidation of ethanol with NO3− as the oxidant is because of the large yield of energy released C) the oxidation of ethanol with oxygen, because the Δ G is the largest negative number indicating the large yield of energy released. D) The oxidation of ethanol yields too little biomass to be catabolized. E) None-ethanol can only be catabolized syntrophically." class="answers-bank-image d-inline" loading="lazy" >A) the catabolism of ethanol without an oxidant, because the Δ G is almost positive
B) the oxidation of ethanol with NO3− as the oxidant is because of the large yield of energy released
C) the oxidation of ethanol with oxygen, because the Δ G is the largest negative number indicating the large yield of energy released.
D) The oxidation of ethanol yields too little biomass to be catabolized.
E) None-ethanol can only be catabolized syntrophically.
Answer