Question 3
(Multiple Choice)
Using the table below, what is the best method for obtaining energy from catabolizing ethanol, and why? A.
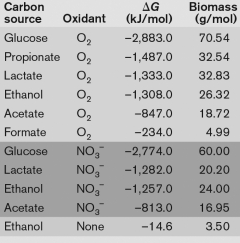 G is almost positive B) the oxidation of ethanol with as the oxidant is because of the large yield of energy released C) the oxidation of ethanol with oxygen, because the G is the largest negative number indicating the large yield of energy released. D) The oxidation of ethanol yields too little biomass to be catabolized. E) None-ethanol can only be catabolized syntrophically." class="answers-bank-image d-inline" loading="lazy" >
G is almost positive B) the oxidation of ethanol with as the oxidant is because of the large yield of energy released C) the oxidation of ethanol with oxygen, because the G is the largest negative number indicating the large yield of energy released. D) The oxidation of ethanol yields too little biomass to be catabolized. E) None-ethanol can only be catabolized syntrophically." class="answers-bank-image d-inline" loading="lazy" >
A) the catabolism of ethanol without an oxidant, because the G is almost positive
B) the oxidation of ethanol with as the oxidant is because of the large yield of energy released
C) the oxidation of ethanol with oxygen, because the G is the largest negative number indicating the large yield of energy released.
D) The oxidation of ethanol yields too little biomass to be catabolized.
E) None-ethanol can only be catabolized syntrophically.
Answer
D