Question 80
(Multiple Choice)
The following questions are based on the reaction A + B ↔ C + D shown in the figure below.
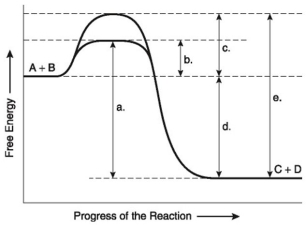
-Which of the following terms best describes the forward reaction in the figure above?
A)endergonic, ∆G > 0
B)exergonic, ∆G < 0
C)endergonic, ∆G < 0
D)exergonic, ∆G > 0
E)chemical equilibrium, ∆G = 0
Answer