Question 48
(Multiple Choice)
The solutions in the arms of a U-tube are separated at the bottom of the tube by a selectively permeable membrane.The membrane is permeable to sodium chloride but not to glucose.Side A is filled with a solution of 0.4 M glucose and 0.5 M sodium chloride (NaCl),and side B is filled with a solution containing 0.8 M glucose and 0.4 M sodium chloride.Initially,the volume in both arms is the same.Refer to the figure to answer the following questions.
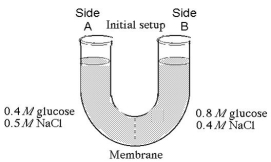
-At the beginning of the experiment,
A)side A is hypertonic to side B.
B)side A is hypotonic to side B.
C)side A is isotonic to side B.
D)side A is hypertonic to side B with respect to glucose.
E)side A is hypotonic to side B with respect to sodium chloride.
Answer