Question 45
(Multiple Choice)
Use the following information to answer the questions below.
The solutions in the two arms of this U-tube are separated by a membrane that is permeable to water and glucose but not to sucrose.Side A is half-filled with a solution of 2 M sucrose and 1 M glucose.Side B is half-filled with 1 M sucrose and 2 M glucose.Initially,the liquid levels on both sides are equal.
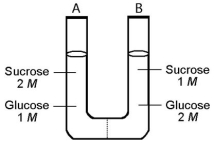
-After the system reaches equilibrium,what changes are observed?
A)The molarity of sucrose and glucose are equal on both sides.
B)The molarity of glucose is higher in side A than in side B.
C)The water level is higher in side A than in side B.
D)The water level is unchanged.
E)The water level is higher in side B than in side A.
Answer