Question 72
(Multiple Choice)
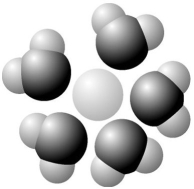
-Based on your knowledge of the polarity of water molecules,the solute molecule depicted here is most likely
A)positively charged.
B)negatively charged.
C)without charge.
D)hydrophobic.
E)nonpolar.
Answer