Question 63
(Multiple Choice)
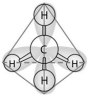
-In the methane molecule shown in the figure above,bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon.The electron orbitals in these bonds are said to be
A)double orbitals.
B)tetrahedral orbitals.
C)complex orbitals.
D)hybrid orbitals.
E)polar orbitals.
Answer