Question 73
(Multiple Choice)
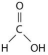
-The illustration above shows a representation of formic acid.A formic acid molecule
A)will form hydrogen bonds with water molecules.
B)has a tetrahedral configuration of hybrid electron orbitals for the carbon atom.
C)consists of largely nonpolar covalent bonds.
D)is held together by hydrogen bonds.
E)has a tetrahedral shape and will form hydrogen bonds with water molecules.
Answer