Question 32
(Short Answer)
The figure below shows anaerobic corrosion of a piece of steel accelerated by the action of anaerobic microorganisms. What should be on label 2? 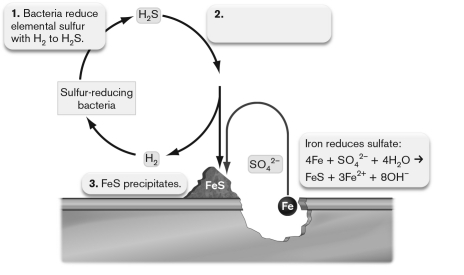 . b. Iron reduces c. Acid rain is produced: d. Acid rain is produced: e. Acid rain is produced: " class="answers-bank-image d-block" loading="lazy" >
. b. Iron reduces c. Acid rain is produced: d. Acid rain is produced: e. Acid rain is produced: " class="answers-bank-image d-block" loading="lazy" >
a. Elemental sulfur and iron directly react to produce .
b. Iron reduces
c. Acid rain is produced:
d. Acid rain is produced:
e. Acid rain is produced:
Answer