Question 41
(Multiple Choice)
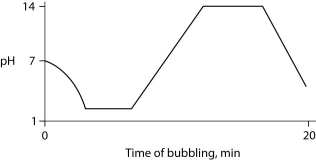
Carbon dioxide (CO₂)is readily soluble in water, according to the equation CO₂ + H₂O ↔ H₂CO₃. Carbonic acid (H₂CO₃)is a weak acid. If CO₂ is bubbled into a beaker containing pure, freshly distilled water, which of the following graphs correctly describes the results?
A)
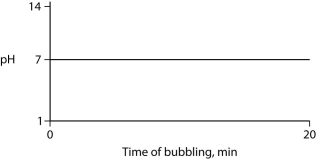
B)
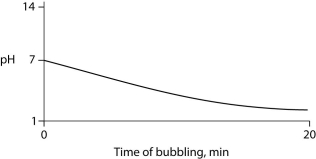
C)
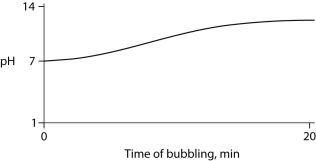
D)
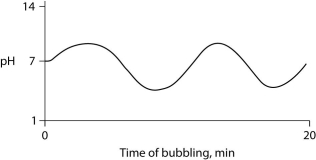
E)
Answer