Question 51
(Multiple Choice)
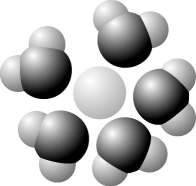
-Based on your knowledge of the polarity of water molecules, the solute molecule depicted here is most likely
A) positively charged.
B) negatively charged.
C) without charge.
D) hydrophobic.
E) nonpolar.
Answer