Question 61
(Multiple Choice)
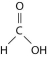
-The illustration above shows a representation of formic acid. A formic acid molecule
A) will form hydrogen bonds with water molecules.
B) has a tetrahedral configuration of hybrid electron orbitals for the carbon atom.
C) consists of largely nonpolar covalent bonds.
D) is held together by hydrogen bonds.
E) has a tetrahedral shape and will form hydrogen bonds with water molecules.
Answer